
Kaloba Pelargonium Cough & Cold Relief Tablets
Single pack £8.40 (RRP: £9.99) - Save 16%
Twin pack £15.00 (RRP: £19.98) - Save 25%
Free UK P&P for orders over £25.00!
Pelargonium sidoides root extract 20mg
30 film-coated tablets
Traditionally used for:
- Common cold
- Sore throat
- Cough
- Runny nose
- Blocked nose
Kaloba Pelargonium Cough and Cold Relief Tablets. A traditional herbal medicinal product used to relieve the symptoms of upper respiratory tract infections including the common cold, such as sore throat, cough and blocked or runny nose, exclusively based on long standing use as a traditional remedy. Always read the label.
| Herbal Active | Pelargonium (Pelargonium sidoides DC.) |
| Strength | 20mg of dried root extract |
| Dose | Adults & children over 12 years: One tablet 3 times daily |
Kaloba contains Pelargonium sidoides, a member of the geranium family. It has long stalked leaves that are mildly aromatic, heart shaped and velvety.
Each pharmaceutical grade coated tablet is foiled blister packed to retain its freshness and maintain its potency.
Adults and children 12+ should take one tablet three times a day (morning, midday, evening). Tablets should be swallowed whole with a little water. The tablets should not be chewed.
After relief of symptoms, continuation of treatment is recommended for a further 2–3 days in order to prevent a relapse. However, treatment duration should not exceed 2 weeks.
A licensed herbal medicine means a safe, high quality product
Kaloba Pelargonium Cough & Cold Relief Tablets have been registered under the Traditional Herbal Registration Scheme (THRS), a regulatory approval process for herbal treatments in the EU. Registration under this scheme primarily means that:
- Kaloba is a regulated herbal product and meets specific standards of safety and quality
- Kaloba is of pharmaceutical quality and has been manufactured to European Good Manufacturing Practice (GMP) Guidelines
The quality of many herbal products on the UK market at present is unknown and there are no independent quality and safety checks available to offer you reassurance about these products. Kaloba’s registration under the THRS scheme provides you with the reassurance that it has been assessed by the MHRA and meets the required standard of safety and quality, as well as providing reliable patient information. The THR number on product packaging provides the proof of evidence that Kaloba has met the MHRA’s agreed level of safety and quality, and that the on-pack consumer information has been approved by the MHRA.
We are committed to improving people's health and wellbeing and do so using only the finest plant based, natural healthcare products. Please click Trust Schwabe for more information about our company.
As seen on TV!
Using Kaloba
What this product is and what it is used for
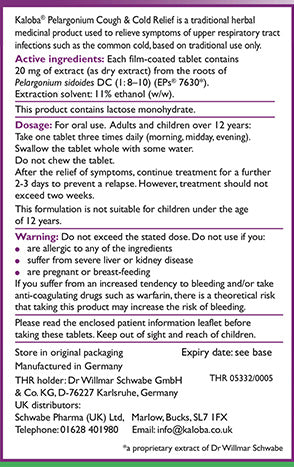
This product is a traditional herbal medicinal product containing Pelargonium sidoides DC root extract. Each film-coated tablet of this product contains 20 mg of a dry extract from the roots of Pelargonium sidoides DC.
Kaloba Pelargonium Cough & Cold Relief is a traditional herbal medicinal product used to relieve the symptoms of upper respiratory tract infections including the common cold, such as sore throat, cough and blocked or runny nose, based on traditional use only.
Before you take this product
DO NOT TAKE this product if you:
- are allergic to any of the ingredients
- are lactose-intolerant (react badly to lactose or milk)
- suffer from severe liver disease
- are pregnant or breast-feeding
This formulation is not suitable for children under the age of 12 years.
How to take this product
For oral use only.
Adults and children over 12 years
Take 1 tablet three times daily (morning, midday, evening).
Swallow the tablet whole with some water. Do not chew the tablet.
After the relief of symptoms, continue treatment for a further 2-3 days to prevent a relapse. However, treatment should not exceed two weeks.
Do not exceed the stated dose.
This formulation is not suitable for children under the age of 12 years.
If you take too much of this product (overdose)
If you take more than the recommended dose, speak to your doctor, pharmacist or qualified healthcare practitioner and take this leaflet with you.
If you forget to take this product
Do not take twice the dose, but continue to take your usual dose at the usual time.
If you have any questions, or are unsure about anything, please ask your doctor, pharmacist or qualified healthcare practitioner.
Possible side-effects
Like all medicines, this product can have side-effects, although not everybody gets them. They are listed below.
Uncommon side-effects (affecting more than 1 in 1,000 but fewer than 1 in 100 people)
Gastro-intestinal complaints such as stomach pain, heartburn, nausea, vomiting, difficulty swallowing (dysphagia) or diarrhoea.
Rare side-effects (affecting more than 1 in 10,000 but fewer than 1 in 1000 people)
- mild bleeding from the gums or nose
- hypersensitivity reactions, e.g.skin rash, nettle rash, itching of the skin and mucous membranes
Very rare side-effects (affecting 1 or fewer in 10,000 people)
Serious hypersensitivity reactions with swelling of the face, breathlessness and decrease in blood pressure.
Liver problems including hepatitis have been reported when taking this product. However, a causal relationship has not been established. The frequency is not known.
After taking this product
Consult a doctor immediately if your condition worsens, or does not improve within one week, in case of fever lasting for several days or in case of shortness of breath or blood in the sputum.
Liver problems including hepatitis have been reported when taking this product. However, a causal relationship has not been established. The frequency is not known. If you become unwell (yellowing eyes/skin, nausea, vomiting, dark urine, abdominal pain, unusual tiredness, loss of appetite), stop taking immediately and seek medical advice.
Tell your doctor or pharmacist if any of the above side-effects becomes serious or if you notice any other side-effects not listed above.
Reporting of side-effects
If you get any side-effects, talk to your doctor, pharmacist or nurse. This includes any possible side-effects not listed in this leaflet. You can also report side-effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side-effects you can help provide more information on the safety of this medicine.
How to store this product
Do not use this product after the expiry date. Return any out-of-date product to your pharmacist who will dispose of it for you. The expiry date is printed on the box and also the blister pack.
Store the product in a cool dry place.
Keep the product out of the reach and sight of children.
Further information Each film-coated tablet contains 20 mg of extract (as dry extract) from the roots of Pelargonium sidoides DC (1: 8–10) (EPs® 7630). Extraction solvent: 11% ethanol (w/w).
This product also contains the following ingredients:
Extract: Maltodextrin
Tablet core: Maltodextrin, microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, precipitated silica, magnesium stearate.
Film-coating: Hypromellose 5 mPas, Macrogol 1500, iron oxide yellow E 172, iron oxide red E 172, titanium dioxide E 171, talc, simeticone, methylcellulose, sorbic acid.
If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this product. Each film-coated tablet contains 20mg of lactose.
Each pack contains 16, 21 or 30 film-coated tablets. Not all pack sizes may be marketed.
Registration holder and manufacturer of this product
Dr Willmar Schwabe GmbH & Co. KG Willmar-Schwabe-Str. 4, 76227 Karlsruhe, Germany
Distributor of this product in the UK
Schwabe Pharma (UK) Ltd Alexander House, Mere Park, Dedmere Road, Marlow, Bucks SL7 1FX
Traditional herbal registration number: THR 05332/0005
