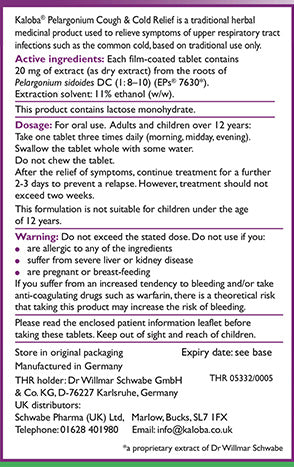
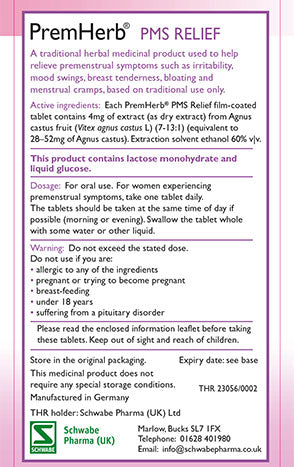
What are THR medicines?
Our herbal products are registered under the Traditional Herbal Registration Scheme which means that they are of pharmaceutical quality and meet specific safety and quality standards based on traditional usage:
- Herbal products are regulated and meet specific standards of safety and quality based on traditional usage
- Products must be of pharmaceutical quality and also be manufactured to European Good Manufacturing Practice (GMP) Guidelines
- All herbal medicines registered under this scheme have a nine-digit registration number on their packaging starting with the letters THR
Not all herbal medicines are created equally
Traditional Herbal Medicines (THRs) are regulated by the Medicines and Healthcare products Regulatory Authority (MHRA) to ensure they comply with safety and quality standards, include clear Patient Information, and are authorised for sale in the UK.
At Schwabe Pharma UK we are proud that all of our herbal medicines meet these licensing requirements: look out for the THR logo on products.
If you want to find more information about Traditional Herbal Medicines you can watch a video made by the British Herbal Medicine Association (BHMA).
Traditional Use – what this means under the THRS
A herbal medicine registered under THRS can make medicinal claims on the basis of evidence of traditional use. This means that the licence holder must show the safety of their herbal medicine by providing bibliographic proof of at least 30 years of long-standing use for the indication – although many products have evidence of hundreds of years of herbal usage.
What does the THRS mean for you, the consumer?
The quality of many herbal products on the UK market at present is unknown and there are no independent quality and safety
checks available to offer consumers reassurance about these products.
The registration of herbal products under the THRS provides this reassurance to consumers and healthcare professionals that
products are assessed by the MHRA (Medicines Health and Regulatory Authority) and meet the required standard of safety and quality, as well as providing reliable patient information.
The THR number on product packaging provides the proof of evidence that a product has met the MHRA’s agreed level of safety and quality, and that the on-pack consumer information has been approved by the MHRA.